Title: An Empirical Model for Stereochemical Control in the Cyclization for Cyclopropanetricarboxylic Acid Esters
URL: https://doi.org/10.1246/bcsj.20190096
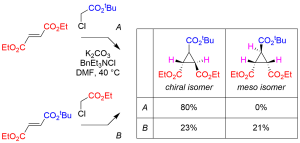
In this paper, we report an empirical model for diastereoselective cyclopropanation of fumarate/maleate diesters with chloroacetate, sulfonium ylide, or ammonium ylide. With symmetrical fumarate/maleate diesters, cyclopropanation was found to proceed with high level of diastereoselectivity in favor of chiral isomer. In contrast, production of meso isomer was observed in 38–48% diastereoselectivity when unsymmetrical fumarate/maleate was employed. An improved synthesis of (N–desmethy)dysibetaine CPa in both racemic and enantiomerically pure forms is also reported.
For structural analysis of cyclopropanes, we also report here usefulness of experimental 13C NMR chemical shift values, in combination with the theoretical data.
Our research has thus shown the usefulness of fumarate/maleate diesters and acetate Michael donor for synthesis design of stereodefined 1,2,3-cyclopropanetricarboxylic acid esters.


