Synthetic Studies on Protoaculeine B
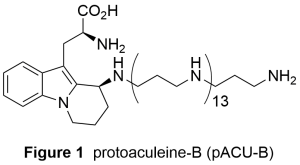
Aculeine B was isolated in 2011 by Sakai et al from marine sponge Axinyssa aculeata collected at Iriomote island, Okinawa, Japan.1 Protoaculeine B (pACU–B, Figure 1) was also isolated from the same sponge as the N–terminal fragment of aculeine B.2 pACU–B is composed of heterocyclic amino acid that would be derived from L–tryptophan and a long chain polyamine (LCPA). pACU–B is expected to have neuroactivity, because some well-known naturally occurring neuroactive agents such as JSTX and PhTX also share amino acid and LCPA. We have started our synthetic study on pACU–B to 1) establish synthetic route that would be also applicable to the analogs and 2) elucidate the action on the neurotransmission.
We decided to focus on the condensation reaction of heterotricycle and LCPA moieties by secondary-amine formation, for which enamide derived from oxime was employed in the present study.
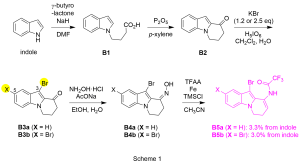
As shown in Scheme 1, starting from indole, coupling with γ–butyrolactone afforded carboxylic acid B1, which was subsequently subjected to cyclization to furnish B2 bearing the third piperidine ring. We next introduced bromo group in order to acquire high reactivity in the N–alkylation. It was found that the number of the bromo group could be controlled by the amount of KBr used and two brominated heterotricycles B3a/B3b were obtained selectively. Heterotricyclic TFA enamides B5a/B5b were then constructed over two steps via oxime B4a/B4b (3.3%/3.0% from indole for 5 steps).
 The key N–alkylation was conducted by alkyl halide and K2CO3 in DMF. Three heterotricyclic TFA enamides were employed. As expected, it was clearly shown that bromo groups enhance the reactivity of the TFA enamides in the N–alkylation. In addition, some TFA enamides were found to be capable of providing even more complex N–alkylation products by the procedure, in acceptable yields.
The key N–alkylation was conducted by alkyl halide and K2CO3 in DMF. Three heterotricyclic TFA enamides were employed. As expected, it was clearly shown that bromo groups enhance the reactivity of the TFA enamides in the N–alkylation. In addition, some TFA enamides were found to be capable of providing even more complex N–alkylation products by the procedure, in acceptable yields.
References
- S. Matsunaga et al, ChemBioChem, 2011, 12, 2191-2200.
- S. Matsunaga et al, Org. Lett. 2014, 16, 3090-3093.