Title: Structure Revision of Protoaculeine B, a Post-translationally Modified N-Terminal Residue in the Peptide Toxin Aculeine B
URL: https://doi.org/10.1021/acs.jnatprod.0c01280
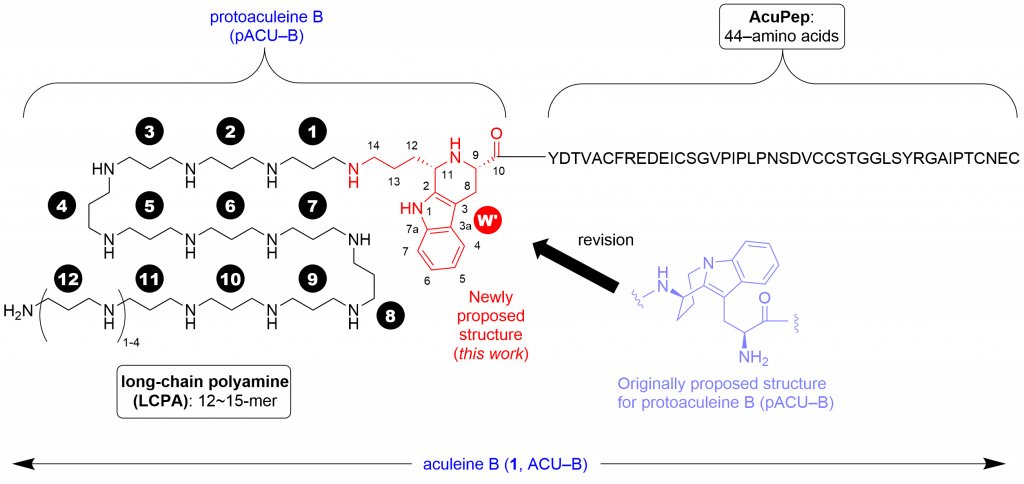
Aculeines (ACUs) A-C are the first example of peptides posttranslationally modified by long-chain polyamine (LCPA) except for silaffin. Toxicity of ACUs to various mammalian cells stems from their ability to disrupt cell membrane, based on the unique but unexplained interactions between ACUs and plasma membrane. To study the mechanism of action of ACUs in detail, we have studied chemical synthesis of ACU-B, and the first synthesis of the proposed structure for protoaculeine B (pACU-B) which corresponds to the N-terminal amino acid residue of ACU-B has been achieved recently (Journal of Natural Products, 2020, 83, 9, 2769–2775; DOI: 10.1021/acs.jnatprod.0c00761). In that paper, we also reported that NMR, mass spectra and chemical reactivity of the synthetic sample were found to differ from those of natural pACU-B, by which we concluded that the originally reported structure requires a revision.
In this paper, we report the cis-disubstituted tetrahydro-beta-carboline (THBC) framework as the true structure for the heterotricyclic moiety of pACU-B.
In this study, we reexamined spectroscopic data of natural pACU-B, and prepared two THBCs that lack long-chain polyamine of pACU-B by the one-step, unprecedented Pictet-Spengler reaction of tryptophan and compared their NMR and mass spectra and chemical reactivity with those of natural pACU-B. The synthetic models reproduced the profiles of the natural product well, which was conclusive of structural revision.
The impact of this study is not limited to structural revision of natural product of biological importance. This study provides insights into strategies for acquiring new biological functions by conjugating peptides with polyamines by posttranslational modification, by the data suggesting that the conjugation is catalyzed by an enzyme (Pictet-Spenglerase). In addition, this research provides important findings on chemical defense by polyamine-modified peptides in nature.


