Title: Four Stereoisomers of 2–Aminomethyl–1–cyclopropanecarboxylic Acid: Synthesis and Biological Evaluation
URL: https://doi.org/10.1246/bcsj.20190168
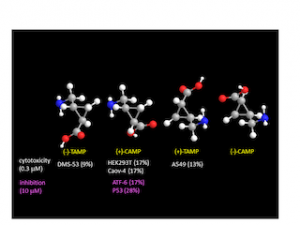
CAMP and TAMP are stereoisomers of 2–aminomethyl–1–cyclopropanecarboxylic acid, which is a GABA analog structurally constrained by cyclopropane ring. In this paper, we developed practical method for asymmetric synthesis of CAMP and TAMP [1], whose oncological activities were further evaluated. We found the activities are weak but specific to cell types, and thus can be the molecular basis of GABA analogs in oncology.
For structural analysis of the synthetic intermediates, we also report here usefulness of experimental 13C NMR chemical shift values, in combination with the theoretical data generated by DFT calculations.
References
[1] For preliminary studies on this work, see; a) M. Oikawa, Y. Sugeno, Y. Ishikawa, H. Tukada, Synlett 2013, 24, 886. b) Y. Sugeno, Y. Ishikawa, M. Oikawa, Synlett 2014, 25, 987.


