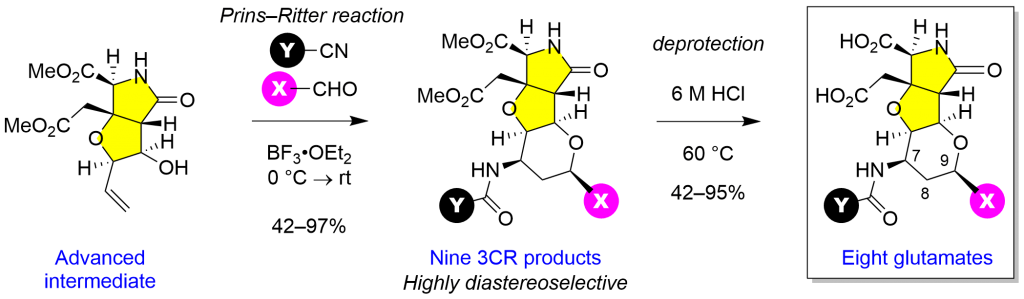
Prins-Ritter reaction of aldehyde, homoallylic alcohol, and nitrile mediated by Lewis acid or Brönsted acid has been used for three-component synthesis of 4–amidotetrahydropyrans in the conventional target-oriented synthesis. However, synthesis of cis-fused heterobicycles has not been explored yet. We show here unprecedented highly diastereoselective Prins–Ritter reaction for synthesis of tetra–cis–substituted 4–amidotetrahydropyrans. In this study, the reaction was not only applied for carbohydrate-based heterobicycles (Scheme 1), but also for more complex heterotricycles (Scheme 2), to show the acceptable levels of conversion yield (42–97% BRSM) and the exclusive diastereoselectivity.
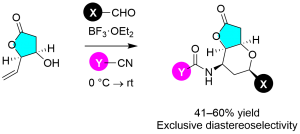 Scheme 1 Prins–Ritter reaction for heterobicycle
Scheme 1 Prins–Ritter reaction for heterobicycle
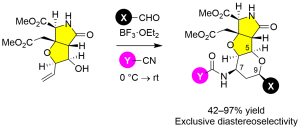 Scheme 2 Prins–Ritter reaction for heterotricycle
Scheme 2 Prins–Ritter reaction for heterotricycle
Furthermore, the latter heterotricycles were led to nine analogs of our ligands for neuronal receptors, IKM–159 and MC–27 (Scheme 3). In vivo assay by intracerebroventricular injection in mice indicated that substituent at C9 interferes molecular interactions with AMPA receptor, which have been observed in the complex of IKM–159 and GluA2 ligand binding domain.
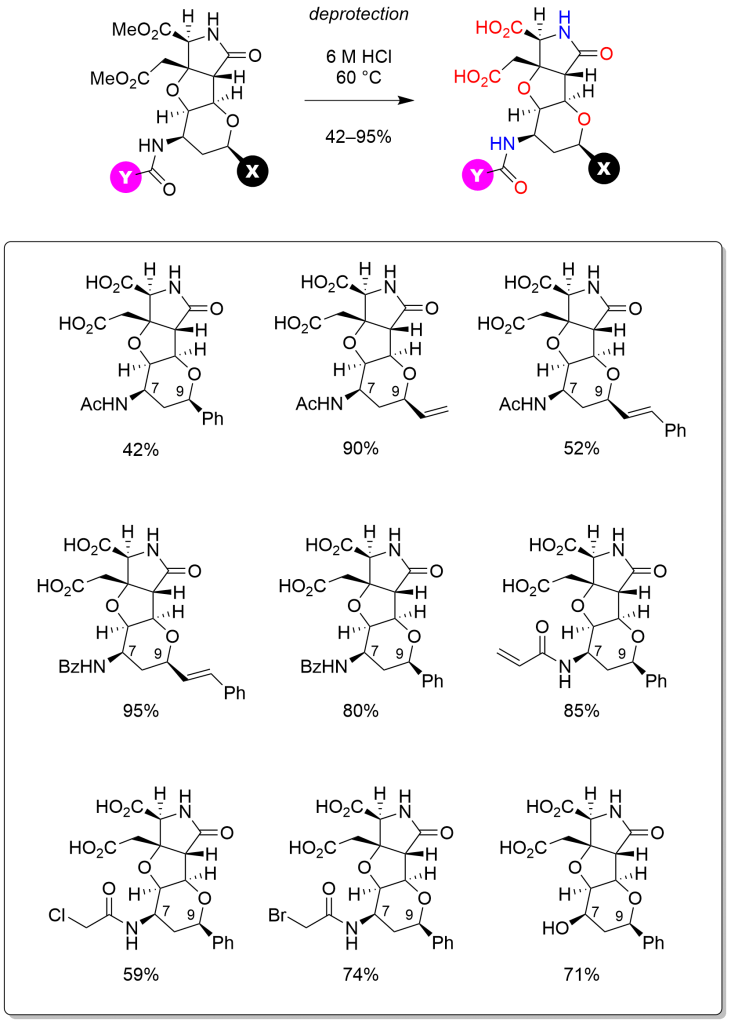
Scheme 3 Final acidic hydrolysis to unveil glutamate motif
Our research has thus shown (1) the structural basis of our neuronal receptor ligands, and (2) the power of multicomponent coupling reaction to explore structure–activity relationships of biologically important small molecules.
The research has been published in ACS Combinatorial Science on May 10, 2016, as a letter.
DOI: 10.1021/acscombsci.6b00046
The ACS Articles on Request e-prints link: http://pubsdc3.acs.org/articlesonrequest/AOR-2fPH8rKicAnD2SkwkFM7