Title: Stereoselective Formation of cis-Trisubstituted 1,3-Dioxanes
URL: https://doi.org/10.1246/cl.210241
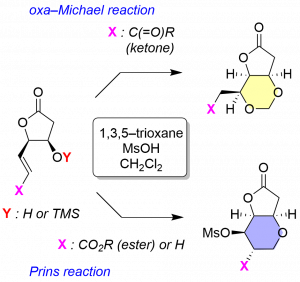
Synthetic heterocyclic compounds are attracting a lot of attention because they provide pharmacophores to more than 90% of new drugs. We have been also studying on selective construction of nitrogen- and oxygen-containing heterocycles to synthesize modulators of neuronal receptors. In this paper, we report construction of cis-trisubstituted, bicyclic 1,3-dioxanes, by hemiacetal formation followed by intramolecular 1,4-conjugate addition (oxa-Michael reaction) on cis-arranged δ-siloxyenones. Using 1,3,5-trioxane and MsOH, the reaction was found to proceed in good yield (81–95%) with complete diastereoselectivity. We furthermore found that stereoselective formation of tetrahydropyran by Prins reaction takes place, when the ester analog (δ-hydroxy unsaturated ester) is exposed to the same combination of the reagents. The methodology reported here is, therefore, useful not only for target-oriented preparation of substituted heterocycles, but also for diversity-oriented synthesis.


